It would be almost impossible to find a better example of
the difficulties that face the pharmaceutical industry than the campaign
against hepatitis C.
Unfortunately, this example is now at the expense of
Vertex Pharmaceuticals, a small, but top-notch drug discovery organization that
began as a biotech startup in 1989.
Vertex had the “good” fortune of becoming the first drug
company to launch a successful antiviral drug, which had a real impact on
hepatitis C—a disease with enormous worldwide public health implications. But,
within a year, their fortunes turned sour.
Called “The Silent Killer,” the viral blood-borne disease
infects the liver, progressively doing irreversible damage over a two- to
three-decade period. Most people who are infected are unaware of this until
symptoms of liver disease show up, at which time the resulting cirrhosis or
liver cancer are life threatening. The majority of
liver transplants in the U.S. are due to liver failure caused by long-term
hepatitis C infection.
Although hepatitis C is much less newsworthy than HIV,
the worldwide infection rate is estimated to be four percent—about four-times
greater than for HIV. With an estimated 150 million infected
people worldwide, the disease became the focus of most infectious disease
research beginning in the mid-1990s—surpassing even HIV in research effort.
Virtually every major drug company launched massive
research campaigns designed to discover specific antiviral therapies to
eradicate the infection. It was far more difficult than anyone could have
imagined.
Although researchers used a very similar strategy that
was supremely successful for HIV, hepatitis C proved to be a much tougher nut
to crack.
Indeed, after a decade of research, and dozens of drug
candidates that failed because of toxicity or lack of efficacy, in 2004,
scientists at Boehringer Ingelheim in Quebec hit the jackpot—or so it seemed. Ciluprevir—the product
of arguably one of the most impressive campaigns in the history of medicinal
chemistry—was shown to reduce the amount of the virus in the blood to nearly
zero after a few doses.
However, unexpected
cardiac toxicity forced the discontinuation of the development of the drug.
Even though Ciluprevir provided the first proof of principle that a specific
antiviral drug could essentially wipe out the virus, this must have been little
comfort for Boehringer.
It would be another seven years until the first HCV drugs
would be approved. Within two weeks, Vertex Pharmaceuticals and Schering-Plough
(now Merck) both launched improved versions of Ciluprevir.
Vertex’s drug, Incivek was superior to Merck’s Victrelis,
and it captured essentially the entire anti-HCV market, with sales of an
astonishing $457 million in its first full quarter.
But
then things went downhill. Given the enormous
(and scientifically brilliant) effort that Vertex put forward to discover
Incivek, it is perfectly fair to expect them to be financially rewarded. But
life is not always fair—especially in the pharmaceutical world.
What went wrong? The efforts
of other companies working in this area
(especially AbbVie, Gilead, Johnson
and Johnson, and Bristol-Myers
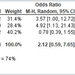
Squibb)—although a bit behind Vertex—were beginning to pay off. Clinical results have been astounding, with cure
rates approaching
100 percent with certain drug combinations. And
aside from the exquisite efficacy of these newer drugs, one enormous advantage
is that interferon—an
injectable immune stimulant that is still part of the standard of care—can be
avoided. This is critical because the side
effects of interferon are so brutal that many patients discontinue treatment,
essentially committing pharmaceutical suicide.
Now, doctors are advising their patients to
wait until some of these second-generation drugs are approved, and this has had a profound impact on Vertex. Sales
have plummeted to $86 million this past quarter, and the
company just announced a staff reduction of 370 employees—15 percent of its
workforce.
This perfectly illustrates two
points: 1) Even when a drug goes through a 10-year approval process and makes
it out the other end, it still may lose money. In fact 70-80 percent of
marketed drugs do just that; 2) Perennial critics of the pharmaceutical
industry, such as Marcia Angell of the Harvard Medical School, who maintain
that second- and third-generation drugs (often derisively ‘called me-too
drugs’) are unnecessary and serve only the companies that make them, must be
living on another planet. Because on this planet the first drug for any given
disease is rarely the best.
Had research and development ceased after
the introduction of Incivek, therapy for hepatitis C would have remained
difficult to endure, and less than optimally effective.
There is no tougher business than drug
discovery. You can win the race and often still come out the loser.
Update 2/11/14: Gilead is now seeking approval for their drug combo consisting of Sovaldi and ledipasvir—two potent anti-HCV drugs that work by different mechanisms (like the AIDS cocktail approach). This will be the first non-interferon therapy for the infection.
Front page image credit: indianexpress.com