A new
patient sits across from me in the exam room, confused and frustrated at her
lack of progress trying to lose weight for the last 30 years. 200 pounds too
heavy, diagnosed with type 2 diabetes, high cholesterol, hypertension, sleep
apnea, infertility and osteoarthritis. She needs knee replacements, but is too
heavy to be approved for the surgery. She asks me, the “weight loss doctor,” what my plan is for
her. And I think:
“What does it take to get referred to a bariatric
surgeon in this town?”
I’ve been interested in
obesity since I began medical practice 14 years ago, but I have to admit that I
believed it to be a lifestyle and behavioral disorder until about 6 years ago,
when I learned about bariatric surgery at an American Diabetes Association
meeting. I was lucky enough to be in a session hosted by some of the pioneering
surgeons and researchers in the field. They described their initial reaction to
the first patient with type 2 diabetes who underwent gastric bypass surgery.
They
had presumed that as the patient lost weight, his metabolism would improve and
that they would eventually be able to reduce or even eliminate the patient’s insulin. What
happened instead is that the patient’s blood sugar normalized and insulin became
unnecessary while he was still in the hospital.
In a
matter of days, the “insulin
resistance” that
was thought to drive type 2 diabetes, as if by magic, resolved. The patient’s natural ability to
sense incoming nutrients, secrete insulin to manage the nutrients, and the body’s ability to accept
the action of insulin, all normalized within the course of a week. The surgeons
were, to say the least, a bit surprised.
Surprised
and excited. So they performed bariatric surgery on a second type 2 diabetic,
then a series of diabetics and observed, over and over again, that the surgery
seemed to reverse the disease process in 80-90% of their patients. The patients
came in on insulin, left a few days later off of it, perhaps never needing it
again. Long before any weight loss occurred.
Dr.
Francesco Rubino, who regaled the audience at the ADA with his tales of rat bypass surgery, showed that type
2 diabetes seems to depend on specific food-gut interactions. He explained that
a bypass works by separating a large portion of the stomach and rerouting the
intestine so that food goes from a smaller stomach directly into the end of the
small intestine. This “bypasses” most of the small
intestine, where a lot of our digestion occurs. It helps with weight loss for
obvious reasons: your stomach is tiny, so you feel full quickly, and if you
eat, it’s
hard to absorb the nutrients, since the small intestine has been bypassed.
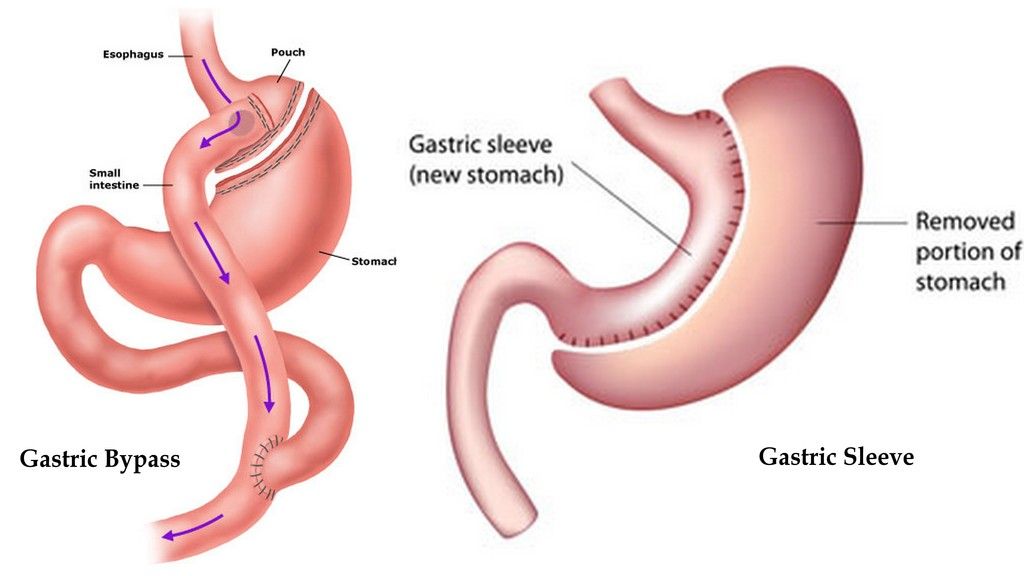
He
performed bypass on rats bred to be obese and diabetic and showed that, just as
in humans, type 2 diabetes went into remission with surgery. He then reversed
the gastric bypass and showed that the diabetes came back. He postulated that
the bypass, by depriving the small intestine of nutrients, was causing a change
in the food-gut-hormone pathways that regulate how we deal with food energy.
To
test this, he stuck tubes down the rats’ throats through the stomach, past the first part of
the intestine and found that it worked just like bypass: diabetes went away,
without the big surgery. He pulled the tube out and the diabetes came back. He
wanted to make sure that it wasn’t just the tube doing something, so he put holes in
the tube, so that food could leak out. The diabetes came back. Back and forth,
over and over again, in these (he called them) heroic rats, with diabetes
getting switched on and off, depending upon whether nutrients came in contact
with the small intestine.
Today,
these results have been reproduced everywhere that bariatric surgery is
systematically studied. Gastric bypass as a "cure" for diabetes is
commonplace (though many doctors seem not to know it). But the results seem to vary
by the method of surgery. If one compares gastric bypass to gastric banding (in
which the stomach is constricted by the band into a small pouch similar to
bypass, but is otherwise left intact) the banding patients do not have nearly
half the luck regarding diabetes resolution, and it seems dependent on weight
loss. This would seem to make it obvious that Dr. Rubino and others were right
when they considered diabetes to be caused by the interaction of nutrients with
the duodenum (first part of the intestine).
However,
gastric “sleeve” bypass, which cuts
away a lot of tissue, leaving only a sleeve shaped stomach to receive food, but
without bypassing the duodenum, does almost as well as the traditional “Roux-en-Y” bypass. Logically,
the sleeve bypass, which leaves one hooked up end to end without disruption,
should do no better than banding if the cause of diabetes is simply food
touching the duodenum.
In a
2011 article in the Journal of Physiology, Erik Hansen and colleagues built
upon the work of these pioneering surgeons by looking at the body’s hormonal
response to bypass surgery. Their goal was to test whether the food actually
touching the duodenum was the direct cause of diabetes remission in bypass
patients. They came up with a clever way to examine this idea. They checked
patients before and after bypass with regard to blood sugar, insulin and other
related hormones. They checked how the patients’ bodies reacted to food delivered to different parts of
the digestive system by inserting a tube into the stomach, connected through
the skin to the outside world, at the time of surgery.
This
way, the study subjects could eat food by mouth, have it travel down the bypass
route straight to the jejunum (missing the duodenum), or, have food put into
the gastric tube connected to the stomach left after surgery (note in the
diagram above, that the stomach remains in the body after Roux En Y bypass).
This second way of feeding would put the food through the old route of stomach,
then duodenum, etc. So the subjects could act as their own controls in an
experiment that compared a human reaction to the same food delivered in three
ways: before surgery with normal anatomy, after surgery using bypassed anatomy
and after surgery using a tube that would still have food pass through the
entire small intestine (which is not removed during bypass, as it is needed to
transport bile from the liver and digestive enzymes from the pancreas). What
did they find?
For
those of us who thought Dr. Rubino's rat experiments explained type 2 diabetes,
as I did, the results were surprising. The patients all had improvements in
blood sugar, insulin secretion, insulin sensitivity and other hormone response
to food. But the route of food administered to the subjects made no
difference. Whether food came in the stomach and touched the duodenum, or
was eaten to bypass the duodenum, the patients appeared to be sensitive to
insulin again. This argues that something else happens during or shortly after
bariatric surgery to cause the change in insulin sensitivity. The surgery
itself changed the way the subjects responded to food later, regardless of
whether or not the food touched the duodenum.
In
2012, two papers in the New England Journal of Medicine made headlines for
reporting diabetes remission rates between 37% and 95% with bariatric surgery
(Schauer and colleagues, Mingrone and colleagues, both on 3/29/12). Previous
accounts of diabetes remission after bypass were primarily observational. That
is, they simply reported the outcomes for the patients that they operated on.
They were without randomization and comparison groups. Based on those earlier
studies, the consensus has been that type 2 diabetes is reversible by surgery
with the following caveats:
The shorter the duration of diabetes, the better
The milder the case, the better
Patients on insulin are harder to “cure”
The more drastic the surgery, the better
Roux –en-Y is best for diabetes, then gastric sleeve, then
gastric banding.
The
New England Journal studies improved upon our understanding of these questions
in three ways. First, by comparing the surgery patients to conventional medical
treatment groups, both studies were able to quantify the degree of improvement
expected by surgery, which previously was simply assumed. Second, by taking
patients with greater duration and worse disease, the Schauer study gave
surgeons a more realistic view of how patients in a real bariatric practice can
expect to fare. Third, the Mingrone study confirmed that bilio-pancreatic
diversion is better than traditional Roux-en-Y bypass in a study with a control
group.
The
Schauer study was conservative in its conclusions, I think to a fault. By
reporting the percentage of patients reaching a target hemoglobin A1C (a
measure of average blood sugar over months) of less than 6% as the definition
of remission of diabetes, they downplay the fact that the vast majority of
surgery patients had tremendous improvement, stopped nearly all medications and
came very close to complete remission in one year. The 37% and 42% remission
reported tends to understate that improvement. The tables included in the
article make clear that one could state with confidence that surgery will
drastically improve diabetes, probably make all medicines unnecessary and
nearly guarantee a major change in the risk of long term complications such as
heart disease and renal failure.
The
trouble with both of these studies is that the comparison group was “conventional medical
therapy” (despite
the fact that Schauer and colleagues termed it “intensive”). What does conventional medical therapy mean? This
means piling one medicine on top of another to reach blood sugar targets and to
give half-hearted advice about lifestyle changes.
What
do I mean by “half-hearted?” How about, “encouraged to
participate in the Weight Watchers program?” To me, that’s about as half-hearted as it gets. The diet advice
was perhaps just slightly worse than what you might get from your neighbor…In my clinical experience,
very few candidates for bariatric surgery have not tried Weight Watchers or
other commercial programs, repeatedly.
So
what would be a good comparison group?
In “Reversal of type 2
diabetes,” published
in Diabetologia in 2011, E.L. Lim and colleagues tested whether eating like a
bariatric surgery patient would have the same effect as actually having the
surgery. In short: they starved their patients without first operating. This
was set up to test what exactly needs to happen for diabetes to disappear. Is
it the nerves or hormonal changes that occur with the operation? Is it altering
the food/gut interaction? Could it simply be that you never eat much again?
They
took 11 volunteers (people don’t line up for starvation studies) with type 2 diabetes
and fed them a diet of 600 calories per day for eight weeks. They found that
after one week of dramatically reduced calorie intake, blood glucose levels
entirely normalized in these diabetic patients. They also showed over the eight
weeks that fatty liver and fatty pancreas (one of the keys to type 2 pathology)
were entirely resolved. The patients were secreting a normal amount of insulin
and their insulin resistance resolved as well. It was as if the patients had
had surgery.
Keep
in mind that this was one hundred year old news when it came out. The original
treatment of diabetes, both types, was starvation and/or complete carbohydrate
restriction.
Is
this a fair comparison? Starvation vs. bariatric surgery?
It’s a better comparison
than “conventional
medical therapy.” I’ve worked in the
bariatric surgeon’s
office and compared notes with the surgery dietitian. We’ve shared pictures of
the meals eaten by successful surgery patients and successful weight loss
patients in my clinic. Believe me, successful bariatric surgery patients are
eating very little, perhaps not much more than the starvation patients in the
Lim study. When surgery patients, over time, increase calories, one, five, ten
years out, the weight begins to creep back. When surgery patients are in their
first year of recovery and the weight is coming off effortlessly, they don’t seem to regain a
normal metabolism so much as they seem to be able to very successfully follow a
crazy rigid diet without any hunger. It makes them, to my mind, wired like a
naturally skinny person: easily full, kind of nauseous with overeating and
prone to eat more nutritive foods. They can even resemble anorexia nervosa
patients.
Perhaps
there is no mystery as to why the surgeries, such as gastric sleeve, that leave
the anatomy intact, are still quite useful for diabetes. Because of the
surgery, food is drastically reduced. In addition, to avoid protein starvation,
post-surgery patients are counseled (almost hounded) by the team's dietitians
to meet protein goals before all else. It may actually be one of the highest
protein and lowest carb diet programs out there.
With
regard to long term follow up, surgery remains the most effective treatment we
have for diabetes. However, medical physicians are generally reluctant to
recommend this drastic approach to a disease that can be well managed with
medications. In addition, every physician has patients or knows of cases who
have had the surgery and found only temporary relief of weight and diabetes.
These exceptions to the rule tend to make an impression, as they confirm a
natural bias against unnecessary surgery. This is made worse by the track
record of early surgical programs which took the heaviest, sickest patients for
bypass first and, not surprisingly, had poor outcomes, including high mortality
rates.
Since
best practices and quality standards became widespread between 2000-2010, the
mortality and other complications from gastric bypass have plummeted and the
safety concerns are inaccurate. An analysis from Dimick and colleagues
published in JAMA in 2013 showed the mortality risk from gastric bypass to be
equivalent to that of removing a gallbladder. To my way of thinking, our
reluctance to consider gastric bypass in our patients may reflect an ongoing
fat bias and an old fashioned understanding of the disease: that it is caused
by gluttony and can be cured by willpower. Once we drop this way of thinking,
we should look for the most effective treatment available for our patients.
With
the "sleeve" gastric bypass, there is nothing particularly
"drastic" about the procedure. The operation takes less than one
hour, it is performed through scope incisions and blood loss is generally
negligible. Yet physicians remain skeptical, pointing to the few exceptions
they've seen. When I discuss this with patients, I point out that we don't stop
sending people for knee replacement because we know of one or two patients who
continued to experience pain, or needed re-operation, or even had blood clots
and died. We consider these unfortunate outcomes as the rare, but possible,
risks of any major surgery and believe that the benefits outweigh the risks.
With regard to bariatric surgery, the irony is that we do continue to refer
those same patients for knee replacements and other procedures with worse risk
profiles than gastric bypass.
We
are only reluctant to send them for the one procedure that might lower their
long term risks for bad outcomes from any procedure.
The
early operations didn't kill patients because the surgery was risky. It was
risky to operate on those patients for any reason. The improvement in mortality
that's occurred over the last twenty years reflects, in part, surgeons
selecting safer patients to operate on, rather than improvement in the surgical
techniques.
In
addition to a primary physician's lack of updated knowledge regarding the risks
of bariatric surgery, there is (again, unfounded) belief that the improvement
in weight and diabetes with bariatric surgery is temporary. After five or ten
years, some physicians believe, the diabetes comes right back. To assess this
question, David Arterburn and colleagues followed over 4,000 gastric bypass
patients for 10 years to look at the longer term results. Their study,
published in Obesity Surgery in 2013, found 68% of previously diabetic bypass
patients to be diabetes-free at 5 years and 40% to remain so at 10 years. To my
way of thinking, this is a report of one of the major triumphs in modern
medicine. While surgery can't be thought
of as a universal cure for diabetes, after surgery most medications, most
especially injections with insulin, will be unnecessary, and the disease will
almost certainly become easily managed, with minimal medication.
Lest
we get too enthusiastic about a surgical cure, there are some important caveats
to consider: First, while the mortality rate of bypass surgery has been
dramatically reduced, there are still post-operative risks that come with
sudden weight loss, starvation type eating, and poor absorption of nutrients.
After surgery, deficiencies in iron, albumin, calcium and vitamin D are
commonplace (as reported in a recent Lancet review, May, 2015, Ikramuddin and
colleagues). Bariatric surgery patients require supplementation and lifelong
monitoring of vitamin levels. When surgery is performed on individuals unable
or unwilling to be vigilant about protein intake, this deficiency will cause
muscle wasting and hair loss. In my practice, I was usually able to spot
patients who had had previous bariatric surgery by the distinct lack of normal
muscle contour around the shoulders. Additionally, surgery patients never get
to enjoy food in the same way. The surgery forces tiny, very frequent feedings,
that need to be followed without the normal call and response of hunger and
satiety. The general recommendation is to never eat and drink at the same
sitting, as the liquid fills the smaller stomach and does not allow enough food
to be consumed. Alcohol is considered off limits because fast absorption makes
its effects felt much more quickly and severely and addictions are more common
after the surgery. It is safe to say that you would not wish this surgery on
someone if they had other choices.
Obesity
and diabetes are not the same. When it comes to type 2 diabetes, I think it is
fair to say that patients have some good choices. Starting metformin early,
adding other oral medications when and if glucose is not controlled, utilizing
newer injectable medications and insulin when required provide a host of
opportunities to keep type 2 diabetes from causing serious harm. Surgery should
continue to be reserved for when these measures fail. But those measures do
fail, quite often. Either through lack of ability on the patient's part to
stick to monitoring and strict medication adherence, or due to the severity of
the disease, many type 2 diabetic patients go on to have amputations, heart
attacks and kidney failure. Earlier consideration of surgery would prevent many
of those secondary consequences.
At
meetings for bariatric societies, it is commonly stated that less than one
percent of individuals who could qualify for gastric bypass surgery are having
the procedure done. "Qualify"would mean, in this case, that a
person's BMI is over 40 (generally 100 pounds overweight) or have a BMI of 35
with diabetes. If a person fits those criteria, society guidelines and
insurance are in favor of surgery. If we take the one percent number at face
value and consider next steps, where does that lead?
If
we were to recognize that bariatric surgery is vastly under-utilized,
mis-understood and much more effective at treating obesity and type 2 DM than
anything else, we might consider trying to put a dent in the 99% of the
population that's not having surgery. To make any real progress, we could
advocate for developing ten times as many bariatric surgery centers as we
currently have. In Iowa, where I live, this would mean that, instead of 5
centers, we would have 50...in more populous states the numbers would be
proportionate, so that in big cities you would see 40-50 bariatric surgery
centers competing to re-route your gut.
Even
if this were feasible, we would then be treating only 10% of those who qualify
for surgery with one of the versions of gastric bypass. 90% of the risk for
kidney failure, heart attacks and amputation would remain. It goes without
saying that we can't scale up another order of magnitude to 400-500 surgery
centers competing in big cities. We will simply have to do something with the
food to get in front of this problem.
Bariatric
surgery is very effective, but also immensely impractical. For me, it is
interesting mostly because of what it tells us about obesity mechanisms and how
diabetes works. I don't think we are ever going to live in a world where
bariatric surgery is commonplace. I think it's much more likely that the
solution to type 2 DM and obesity will occur elsewhere.