For
years it would not have been possible to use the word “silence” in the same
sentence with BPA (bisphenol A). The safety of BPA has been a long-running,
robust controversy, in particular regarding concerns that BPA might cause
health effects at exposure levels in the very low range that we as consumers
might experience every day.
At
times, the loud and ubiquitous discussion on BPA might have best been described
as a cacophony. Lately though, just when
you might expect the volume to reach a crescendo with results from the CLARITY study,
it’s been strangely quiet.
As
with any controversy, there are two sides and, in this case, both are based on
scientific information. Government
agencies, based on comprehensive scientific evaluations, have long considered
BPA to be safe at typical consumer exposure levels.
In
making regulatory decisions, government agencies consider all available
scientific data, but typically give greater weight to studies that follow
internationally accepted guidelines developed specifically for safety testing. In contrast, research conducted by academic
scientists is often exploratory in nature and not specifically aimed at
assessing safety. Much of the
controversy surrounding BPA is due to different views on how to interpret the
results from these different types of studies.
To resolve the impasse, a consortium of
government and academic scientists worked together on a single study that
includes both a core guideline
study and a series of related academic studies. The study is known collectively as CLARITY,
which is an acronym that stands for Consortium Linking Academic and Regulatory Insights on BPA Toxicity.
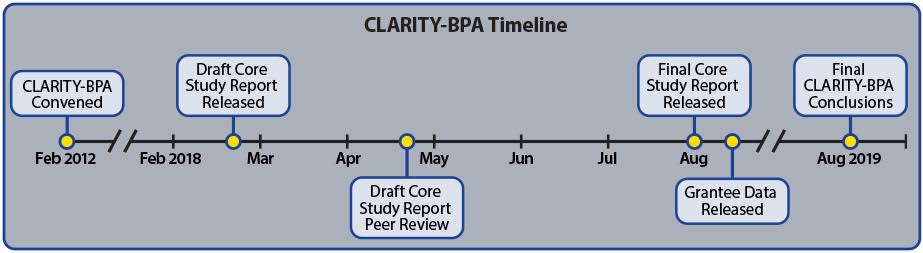
The
results of the guideline study, which was conducted by U.S. Food and Drug
Administration (FDA) scientists in an FDA laboratory, were released earlier
this year in the form of a draft report.
The conclusions
provide strong additional support for the safety of BPA.
What
happened in the academic studies is somewhat of a mystery. So far, results from only five of the 13
academic studies have been published.
Those results, showing minimal effects from BPA, are generally
consistent with the conclusions of the core guideline study. Results from the other eight academic studies
have not yet been published.
Regardless
of whether results are published or not, the silence will end soon and all of
the CLARITY data will speak. One of the
requirements to participate in the CLARITY study was that all researchers,
including the academic researchers, were to upload their raw data into a
database controlled by the U.S. National Toxicology Program (NTP). Public access to all of the data is scheduled
to occur this month, August 2018.
All Studies Are Not Created Equal
Almost
certainly, BPA is one of the best tested substances in commerce. Thousands
of studies of all types have been conducted on BPA by researchers
around the world and many of the studies suggest that BPA may cause health
effects at low levels of exposure, even at the very levels typically
experienced by consumers.
A
challenge for understanding the safety of BPA is that the diversity of studies
is matched by the diversity of results.
Whether you think BPA is safe or not depends to some extent on which
results you choose to believe.
The
many studies on BPA can be roughly sorted into two broad categories. First are studies that follow internationally
accepted guidelines for testing safety.
For example, the OECD
(Organisation for Economic Co-operation and Development) has prepared a series
of guidelines under its chemicals management program for this purpose.
Since
these guidelines are developed and validated by international experts, results
from guideline studies are generally considered to be reliable and relevant for
safety. Numerous guideline studies have
been conducted with results that uniformly support the safety of BPA.
In
comparison, research conducted by academic scientists may not be aimed
specifically at testing safety and typically does not follow accepted
guidelines. Academic research is often
exploratory in nature and it is more difficult to judge whether the results are
both reliable and relevant for safety.
Many academic studies have also been conducted, often with results that
suggest BPA may not be safe.
CLARITY Research Bridges Regulatory
and Exploratory Science
With
no end to the controversy in sight, the CLARITY study was created to bridge
between the guideline and academic studies on BPA. The study was jointly designed by scientists
from two U.S. federal government agencies (FDA and NTP) and 13 academic
scientists who also received funding to support their participation in the
study.
The
core guideline study, which is of unprecedented scope and magnitude for BPA,
was conducted by senior scientists at FDA’s National Center for Toxicological
Research. The results
of the core study were released in February 2018 and have been
peer-reviewed by a panel of independent scientists, with a final report
expected in the near future.
The
data from the core study have spoken very loudly and very clearly. As concluded in the draft study report, “BPA
produced minimal effects that were distinguishable from background.” This prompted FDA to comment in a statement
that “our initial review supports our determination that currently
authorized uses of BPA continue to be safe for consumers.”
The
academic researchers were provided with animals or other biological samples
taken from the core study to ensure that all studies used animals that were
handled in exactly the same way. Results
from five of the academic studies have been published in a series of 11 papers
starting three years ago. Similar to the
core guideline study, minimal effects were found in these studies as well.
The
status of results from the eight missing academic studies is intriguing, to say
the least, and after years of speaking out on BPA, the sudden silence from this
quarter is striking. Following the
protocol they helped to devise, the academic researchers all received animals
or other biological samples between 4.5 and 5.5 years ago. All received federal funds to conduct their
studies, totaling about $8 million for the 13 researchers combined. According to public records on the grants,
all of the academic projects have been complete for some time.
What
is particularly intriguing is that most of the eight researchers who have not
published their results have been critics of BPA in the past, sometimes in a
very vocal way, and presumably would be motivated to report their results. Or at least they would if those results
suggest that BPA is not safe. Why the
silence now after participating in the design of the study and accepting
funding for their studies?
The Data Will Speak
An
important element of the CLARITY study design will ensure that the data will
not be silenced. To the maximum extent
possible, all animals or other biological samples provided to the academic
researchers were coded, meaning that the researchers did not know which dose
group each animal came from.
The
researchers were required to upload their raw data to an NTP database known as
CEBS (Chemical
Effects in Biological Systems) before the code was broken and the dose
group details were disclosed to them.
Only at that point, after the raw data were locked down in the database,
were the researchers able to analyze their data. The purpose for working blind in this way was
to avoid researcher bias.
Although the raw data
is currently locked down in CEBS, it is expected to be opened to the public in
August 2018. At that point, anyone will
be able to download and analyze the data, regardless of whether it has been
published or not. It is now only a
matter of time before the data will speak.