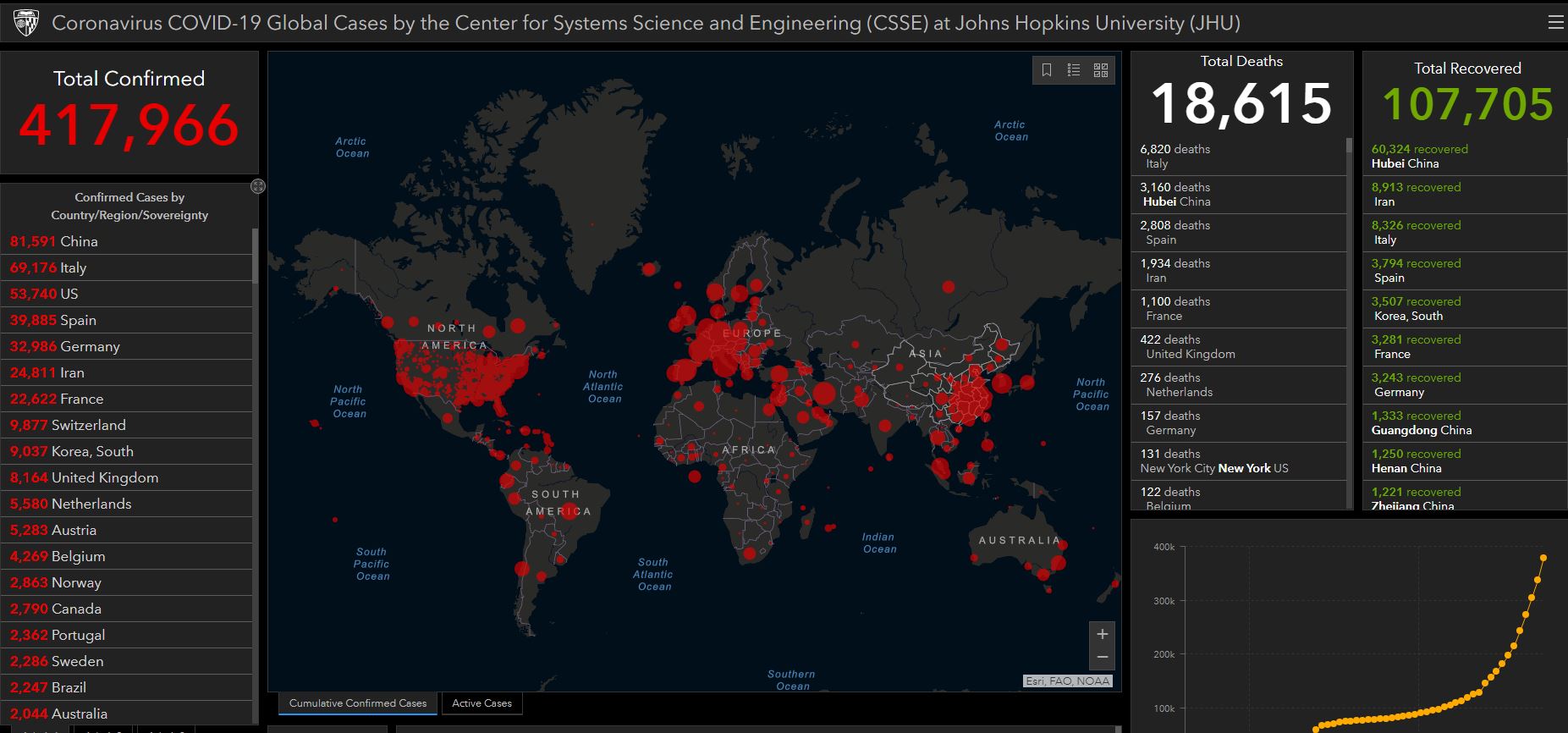
The U.S. Food and Drug Administration continue to be the kind of nimble agency it once was in response to the SARS-CoV-2 coronavirus pandemic, and the hundreds of U.S. deaths COVID-19 has caused.
They are not only facilitating access to antibody-rich plasma donated by people who have recovered from COVID-19, in hopes transfusions could lessen the severity or duration of the illness, they have gone a step further and are helping create a master protocol so independent investigators can work on this without the usual 'I won't share data in case I get beat to publication' concern that besets the life sciences. Actual collaboration.
For now, those willing to study plasma from recovered people can go through the process of single patient emergency Investigational New Drug Applications for individual patients. The agency have also conceded that a whole lot of imported respirators that have not gone through the usual National Institute for Occupational Safety and Health (NIOSH) certification process are fine based on existing evidence from use everywhere else.
It's a breathtaking display of government showing us how useless and self-serving far too many regulations are. Can you imagine the time and cost if there weren't a pandemic? NIOSH alone would take years.
In other areas, they are still a little slow but that may be warranted because of concern about false positives and negatives. Though they have issued 16 emergency use authorizations for testing groups, almost 200 more can do so and will need approval.
And they are having to waste time telling the public not to drink chloroquine phosphate, which treats kills in aquarium fish. It has been in the news, because New York bought 70,000 doses of hydroxychloroquine and 750,000 doses of chloroquine to begin trials. So someone drank it because they heard the President talk about it.
Twitter naturally blamed Trump for that. FDA can't fix the common disease known as political theater.