
I love separation science, since
it amuses me no end. As the coffee stain still lurks at my desk, reading
through this article, the stain will be a mainstay at my laboratory. Okay, the
hygiene issues will linger. Scientists at Harvard, California and Stanford universities
have come up with use of coffee ring effect. A chromatography method that uses
the same physics as the coffee stain: It separates nanometer- and
micrometer-scale particles by size as a droplet dries.
Considering that coffee is a
colloidal suspension, when coffee droplets dry on a clean surface, the
evaporating liquid pushes the particles to the edge, concentrating them and
leaving a ring at the droplet rim.
All this while, we may consider that the
particles just concentrate at the rim of the coffee stain, they are working on theory
as to how the particles move as the droplet evaporates. Here comes in the
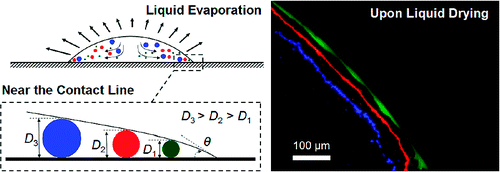
theory of particle separating capacity. As the droplet grew thinner towards its
edge, the particles would stop moving at a point where their diameter matched the
droplet's height.
Courtesy: Analytical Chemistry
They elucidated the theory through an experiment wherein they made an aqueous suspension of fluorescent
beads with diameters of 40 nm, 1 μm, and 2 μm. They let a 0.5-μL droplet of the
suspension dry at room temperature. Three distinct rings appeared, with the
smallest beads in the outermost ring and the largest ones in the innermost
ring. The technique is claimed to separate macromolecules, proteins, bacteria,
and mammalian cells in an aqueous mixture, that too without any use of external
power source.
This technique definitely works
well as I tried to figure out the separation of some amino acids using this theory.
Well, this makes sense especially in a country like India where resources are
scarce. Though, this may raise some hygiene issues, if I allow that stain to remain
on my table, but at this point of time I need some coffee stain.
Coffee Stains…… Anyone !